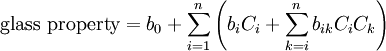Glass
2007 Schools Wikipedia Selection. Related subjects: Materials science
Glass is a uniform material of arguable phase, usually produced when the viscous molten material cools very rapidly to below its glass transition temperature, without sufficient time for a regular crystal lattice to form. The most familiar form of glass is the Silica-based material used for windows.
Glass is a biologically inactive material that can be formed into smooth and impervious surfaces. Glass is brittle and will break into sharp shards. These properties can be modified or changed with the addition of other compounds or heat treatment.
Common glass contains about 70-72 weight % of silicon dioxide (SiO2). The major raw material is sand (or "quartz sand") that contains almost 100% of crystalline silica in the form of quartz. Although it is an almost pure quartz, it may still contain a small amount (< 1%) of iron oxides that would colour the glass, so this sand is usually enriched in the factory to reduce the iron oxide amount to < 0.05%. Large natural single crystals of quartz are purer silicon dioxide, and upon crushing are used for high quality specialty glasses. Synthetic amorphous silica (practically 100% pure) is the raw material for the most expensive specialty glasses.
Properties and uses
The most obvious characteristic of ordinary glass is that it is transparent to visible light (not all glassy materials are). This transparency is due to an absence of electronic transition states in the range of visible light, and because ordinary glass is homogeneous on all length scales greater than about a wavelength of visible light. (Heterogeneities cause light to be scattered, breaking up any coherent image transmission). Ordinary glass partially blocks UVA (wavelength between 400 and 300 nm) and completely blocks UVC and UVB (wavelengths shorter than 300 nm) due to the addition of compounds such as soda ash (sodium carbonate)
Pure SiO2 glass (also called fused quartz) does not absorb UV light and is used for applications that require transparency in this region, although it is more expensive. This type of glass can be made so pure that when made into fibre optic cables, hundreds of kilometres of glass are transparent at infrared wavelengths. Individual fibres are given an equally transparent core of SiO2/GeO2 glass, which has only slightly different optical properties (the germanium contributing to a higher index of refraction). Undersea cables have sections doped with erbium, which amplify transmitted signals by laser emission from within the glass itself. Amorphous SiO2 is also used as a dielectric material in integrated circuits due to the smooth and electrically neutral interface it forms with silicon.
Glasses used for making optical devices are categorized using a six-digit glass code, or alternatively a letter-number code from the Schott Glass catalogue. For example, BK7 is a low- dispersion borosilicate crown glass, and SF10 is a high-dispersion dense flint glass. The glasses are arranged by composition, refractive index, and Abbe number.
Glass is sometimes created naturally from volcanic magma. This glass is called obsidian, and is usually black with impurities. Obsidian is a raw material for flintknappers, who have used it to make extremely sharp knives since the stone age. Collecting obsidian from national parks and other locations may be prohibited by law in some countries, but the same toolmaking techniques can be applied to industrially-made glass.
Glass ingredients
Pure silica (SiO2) has a melting point of about 2,000 ° C (3,632 ° F). It can be made into glass for special applications (see fused quartz), and other substances are added to common glass to simplify processing. One is soda ( sodium carbonate Na2CO3), which lowers the melting point to about 1,000° C (1,832° F). However, the soda makes the glass water soluble, which is usually undesirable, so lime ( calcium oxide, CaO), some MgO and aluminium oxide are added to provide for a better chemical durability. The resulting glass contains about 70 to 72 percent silica by weight and is called a soda-lime glass. Soda-lime glasses account for about 90 percent of manufactured glass.
As well as soda and lime, most common glass has other ingredients added to change its properties. Lead glass, such as lead crystal or flint glass, is more 'brilliant' because the increased refractive index causes noticeably more "sparkles", while boron may be added to change the thermal and electrical properties, as in Pyrex. Adding barium also increases the refractive index. Thorium oxide gives glass a high refractive index and low dispersion, and was formerly used in producing high-quality lenses, but due to its radioactivity has been replaced by lanthanum oxide in modern glasses. Large amounts of iron are used in glass that absorbs infrared energy, such as heat absorbing filters for movie projectors, while cerium(IV) oxide can be used for glass that absorbs UV wavelengths (biologically damaging ionizing radiation).
Glasses that do not include silica as a major constituent are sometimes used for fibre optics and other specialized technical applications. These include fluorozirconate, fluoroaluminate, and chalcogenide glasses.
In 2006 Italian scientists created a new type of glass using extreme pressure and carbon dioxide. The substance was named amorphous carbonia (a-CO2) which has an atomic structure resembling that of ordinary window glass .
Glass as a polymer
An innovative way of making glass involves preparation by polymerization. Putting in additives that modify the properties of glass is problematic, because the high temperature of preparation destroys most of them. By polymerizing glass it is possible to embed active molecules, such as enzymes, to add a new level of functionality to the glass vessels. Sol gel is a very good example of glass prepared in this way.
Colors
Glass appears colorless to the naked eye when it is thin, though it can be seen to be green when it is thick, or with the aid of scientific instruments. However, metals and metal oxides can be added to glass during its manufacture to change its colour.
- Iron(II) oxide results in bluish-green glass, frequently used for beer bottles. Together with chromium it gives a richer green colour, used for wine bottles.
- Sulfur, together with carbon and iron salts, is used to form iron polysulfides and produce amber glass ranging from yellowish to almost black. In borosilicate glasses rich in boron, sulfur imparts a blue colour. With calcium it yields a deep yellow colour.
- Manganese can be added in small amounts to remove the green tint given by iron, or in higher concentrations to give glass an amethyst colour. Manganese is one of the oldest glass additives, and purple manganese glass was used since early Egyptian history.
- Selenium, like manganese, can be used in small concentrations to decolorize glass, or in higher concentrations to impart a reddish colour, caused by selenium atoms dispersed in glass. It is a very important agent to make pink and red glass. When used together with cadmium sulfide , it yields a brilliant red colour known as "Selenium Ruby".
- Small concentrations of cobalt (0.025 to 0.1%) yield blue glass. The best results are achieved when using glass containing potash. Very small amounts can be used for decolorizing.
- Tin oxide with antimony and arsenic oxides produce an opaque white glass, first used in Venice to produce an imitation porcelain.
- 2 to 3% of copper oxide produces a turquoise colour.
- Pure metallic copper produces a very dark red, opaque glass, which is sometimes used as a substitute for gold in the production of ruby-colored glass.
- Nickel, depending on the concentration, produces blue, or violet, or even black glass. Lead crystal with added nickel acquires purplish colour. Nickel together with small amount of cobalt was used for decolorizing of lead glass.
- Chromium is a very powerful colorizing agent, yielding dark green or in higher concentrations even black colour. Together with tin oxide and arsenic it yields emerald green glass. Chromium aventurine, in which aventurescence was achieved by growth of large parallel chromium(III) oxide plates, was also made from glass with added chromium.
- Cadmium together with sulfur results in deep yellow colour, often used in glazes. However, cadmium is toxic.
- Adding titanium produces yellowish- brown glass. Titanium is rarely used on its own, is more often employed to intensify and brighten other colorizing additives.
- Metallic gold, in very small concentrations (around 0.001%), produces a rich ruby-colored glass ("Ruby Gold"), while lower concentrations produces a less intense red, often marketed as " cranberry". The colour is caused by the size and dispersion of gold particles. Ruby gold glass is usually made of lead glass with added tin.
- Uranium (0.1 to 2%) can be added to give glass a fluorescent yellow or green colour . Uranium glass is typically not radioactive enough to be dangerous, but if ground into a powder, such as by polishing with sandpaper, and inhaled, it can be carcinogenic. When used with lead glass with very high proportion of lead, produces a deep red colour.
- Silver compounds (notably silver nitrate) can produce a range of colors from orange-red to yellow. The way the glass is heated and cooled can significantly affect the colors produced by these compounds. The chemistry involved is complex and not well understood.wow
Calculation of Glass Properties
Glass properties can be calculated through statistical analysis of glass databases such as SciGlass and Interglad. If the desired glass property is not related to crystallization (e.g., liquidus temperature) or phase separation linear regression can be applied using polynomial functions up to the third degree. The image below shows an example equation of the second degree. The C-values are the glass component concentrations like Na2O or CaO in percent or other fractions, the b-values are coefficients, and n is the total number of glass components. The glass main component silica (SiO2) is excluded in the equation below because of over-parametrization due to the constraint that all components sum up to 100%. Many terms in the equation below can be neglected based on correlation and significance analysis. Further details and examples are available at Glassproperties.com.
The liquidus temperature has been modeled using neural networks regression in the following article: C. Dreyfus, G. Dreyfus: "A machine learning approach to the estimation of the liquidus temperature of glass-forming oxide blends"; J. Non-Cryst. Solids, vol. 318, 2003, p 63-78.
It is often required to optimize several glass properties simultaneously, including production costs. This can be performed in Microsoft Excel as follows: 1) Listing of the desired properties; 2) Entering of models for the reliable calculation of properties based on the glass composition, including a formula for estimating the production costs; 3) Calculation of the squares of the differences between desired and calculated properties; 4) Reduction of the sum of squares using the Solver option in Microsoft Excel with the glass components as variables. It is possible to weight the desired properties differently. Basic information about the principle can be found in the article: N. T. Huff, A. D. Call: "Computerized Prediction of Glass Compositions from Properties"; J. Am. Ceram. Soc., vol. 56, 1973, p 55-57.
History of glass
Phoenicia and Egypt
Naturally occurring glass, such as obsidian, has been used since the stone age. According to Pliny the Elder, the Phoenicians made the first glass. Pliny wrote: "The tradition is that a merchant ship laden with nitrum (soda and potash) being moored at this place, the merchants were preparing their meal on the beach, and not having stones to prop up their pots, they used lumps of nitrum from the ship, which fused and mixed with the sands of the shore, and there flowed streams of a new translucent liquid, and thus was the origin of glass." That the Phoenicians used glass as a glaze for pottery was known as early as 3000 BC. However, there is archaeological evidence to support the claim that the first glass was made in Mesopotamia. Glass beads, seals, and architectural decorations date from around 2500 B.C.
The color of "natural glass" is green to bluish green. This colour is caused by naturally occurring iron impurities in the sand. Common glass today usually has a slight green or blue tint, arising from these same impurities. Glassmakers learned to make colored glass by adding metallic compounds and mineral oxides to produce brilliant hues of red, green, and blue - the colors of gemstones. When gem-cutters learned to cut glass, they found clear glass was an excellent refractor of light. The earliest known beads from Egypt were made during the New Kingdom, about 1500 BC and came in a variety of colors. They were made by winding molten glass around a metal bar and were highly prized as a trading commodity, especially blue ones because they were reported to have magical powers.
The Egyptians also made small jars and bottles using the core-formed method. Glass threads were wound around a bag of sand tied to a rod and the glass was continually reheated to fuse the threads together. The glass had to be kept in motion until the required shape and thickness was achieved. The final step was to allow the rod to cool then to puncture the bag and remove the rod. The Egyptians also formed the first colored glass rods which they used to create colorful beads and decorations, they also worked with cast glass. . By the 5th century BC this technology had spread to at least Greece. In the first century BC there were many glass centres located around the Mediterranean and at the eastern end of the Mediterranean glass blowing, both free-blowing and mould-blowing, was discovered.
Romans
The advent of the Roman Empire saw the development of many new techniques and as the Empire spread so did the popularity of glass. Through conquest and trade the use of glass objects and the techniques used for making glass were spread as far north as Scandinavia, the British Isles and China. This spreading of technology resulted in glass artists congregating in areas such as
Alexandria in Egypt where the famous Portland Vase was created, the Rhine Valley where Bohemian glass was developed and to Byzantium where glass designs became very ornate and processes such as enamelling, staining and gilding were developed. Window glass was quite commonly used during the 1st century BC, examples found in Karanis, Egypt were translucent and very thick. After the fall of the Empire, the Emperor Constatine moved to Byzantium where the use of glass continued. However, in the rest of the Empire the use of glass declined and many previously known techniques disappeared. Glass didn't completely go out of use, but it didn't become popular again in the west until its resurgence in the 7th century.
Europe
Glass objects from the 7th and 8th centuries have been found on the island of Torcello near Venice. These form an important link between Roman times and the later importance of that city in the production of the material. About 1000 AD, an important technical breakthrough was made in Northern Europe when soda glass was replaced by glass made from a much more readily available material: potash obtained from wood ashes. From this point on, northern glass differed significantly from that made in the Mediterranean area, where soda remained in common use.
The 11th century saw the emergence, in Germany, of new ways of making sheet glass by blowing spheres, swinging these out to form cylinders, cutting these while still hot, and then flattening the sheets. This technique was perfected in 13th century Venice.
The 11th century also saw the emergence of glass mirrors in Islamic Spain.
Until the 12th century, stained glass (i.e., glass with some coloring impurities, usually metals) was not widely used.
The centre for glass making from the 14th century was Venice in the island of Murano, which developed many new techniques and became the centre of a lucrative export trade in dinner ware, mirrors, and other luxury items. What made Venetian Murano Glass significantly different was that the local quartz pebbles were almost pure silica and were ground into a fine clear sand that was combined with another locally occurring product called "Levant soda ash", for which the Venetians held the sole monopoly. This resulted in the Venetians producing a superior form of glass which resulted in them having a trade advantage over other glass producing lands.
History of Murano Glassmaking
Murano’s reputation as a centre for glassmaking was born when the Venetian Republic, fearing fire and destruction to the city’s mostly wood buildings, ordered glassmakers to move their foundries to Murano in 1291. Murano glass is still interwoven with Venetian glass. Murano's glassmakers were soon the island’s most prominent citizens. By the 14th century, glass makers were allowed to wear swords, enjoyed immunity from prosecution by the Venetian state and found their daughters married into Venice’s most affluent families. Of course there was a catch: Glassmakers weren't allowed to leave the Republic. However, many craftsmen took this risk and set up glass furnaces in surrounding cities and as far afield as England and the Netherlands. Murano’s glassmakers held a monopoly on quality glassmaking for centuries, developing or refining many technologies including crystalline glass, enameled glass (smalto), glass with threads of gold (aventurine), multicolored glass (millefiori), milk glass (lattimo), and imitation gemstones made of glass. Today, the artisans of Murano are still employing these century-old techniques, crafting everything from contemporary art glass and glass jewelry to murano glass chandeliers and wine stoppers. The Promovetro Consortium was set up in 1985 to safeguard, promote and defend original glass production on the island of Murano, always a synonym for unique quality and style. The introduction of the Vetro Artistico® Murano mark represents a fundamental milestone for the Consortium which has been its sole administrator in Italy and abroad since 2001. The Consortium is the only body representing the Murano glassworks who can affix the origin mark to their products to make them recognisable on the market in an effort to oppose the numerous attempts at speculation and imitation which damage this Made in Italy symbol. The Consortium, authentic custodian of the Murano art, is committed to defending the glass making tradition of Murano by providing information to the consumer and through initiatives aimed at heightening public awareness of this unique, precious and inimitable product.
English glass was still sufficiently poor that anything a witness saw through a closed window was not admissible as evidence in court until the late 1700s. Eventually some of the Venetian glass workers moved to other areas of northern Europe and glass making spread with them.
The Crown glass process was used up to the mid-1800s. In this process, the glassblower would spin around 9 pound (4 kg) of molten glass at the end of a rod until it flattened into a disk approximately 5 feet (1.5 m) in diameter. The disk would then be cut into panes. Venetian glass was highly prized between the 10th and 14th centuries. Around 1688, a process for casting glass was developed, which led to its becoming a much more commonly used material. The invention of the glass pressing machine in 1827 allowed the mass production of inexpensive glass articles.
The cylinder method of creating flat glass was first used in the United States of America in the 1820s. It was used to commercially produce windows. This and other types of hand-blown sheet glass was replaced in the 20th century by rolled plate.
Glass artifacts
Since glass is strong and non-reactive, it is a very useful material. Many household objects are made of glass. Drinking glasses, bowls, and bottles are often made of glass, as are light bulbs, mirrors, cathode ray tubes, and windows. In laboratories doing research in chemistry, biology, physics and many other fields, flasks, test tubes, lenses and other laboratory equipment are often made of glass. For these applications, borosilicate glass (such as Pyrex) is usually used for its strength and low coefficient of thermal expansion, which gives greater resistance to thermal shock and allows for greater accuracy in laboratory measurements when heating and cooling experiments. For the most demanding applications, quartz glass is used, although it is very difficult to work. Most such glass is mass-produced using various industrial processes, but most large laboratories need so much custom glassware that they keep a glassblower on staff. Volcanic glasses, such as obsidian, have long been used to make stone tools, and flint knapping techniques can easily be adapted to mass-produced glass.
Glass art
Even with the availability of common glassware, hand blown or lampworked glassware remains popular for its artistry. Some artists in glass include Dale Chihuly, Lino Tagliapietra, Kenji Ito, Hans Godo Frabel, Rene Lalique, and Louis Comfort Tiffany, who were responsible for extraordinary glass objects. The term "crystal glass", derived from rock crystal, has come to denote high-grade colorless glass, often containing lead, and is sometimes applied to any fine hand-blown glass such as Edinburgh Crystal and other brands.
Someone who works with hot glass is called a glassblower or lampworker, and these techniques are how most fine glassware is created. Warm glass refers to the technique of manipulating glass in a kiln .
Cold work includes traditional stained glass work as well as other methods of shaping glass at room temperature. Glass can also be cut with a diamond saw, or copper wheels embedded with abrasives, and polished to give gleaming facets; the technique used in creating waterford crystal. Art is sometimes etched into glass via the use of acid, caustic, or abrasive substances. Traditionally this was done after the glass was blown or cast. In the 1920s a new mould-etch process was invented, in which art was etched directly into the mould, so that each cast piece emerged from the mould with the image already on the surface of the glass. This reduced manufacturing costs and, combined with a wider use of colored glass, led to cheap glassware in the 1930s, which later became known as Depression glass. As the types of acids used in this process are extremely hazardous, abrasive methods have gained popularity.
Objects made out of glass include vessels ( bowls, vases, and other containers), paperweights, marbles, beads, smoking pipes, bongs, and sculptures. Colored glass is often used, though sometimes the glass is painted; notable examples of painted glass include the work of contemporary artists Judith Schaechter and Walter Lieberman. Innumerable examples exist of the use of stained glass, such as those by John La Farge in Boston's Trinity Church, or the life-sized sculptures among the fine art of Jim Gary.
The Harvard Museum of Natural History has a collection of extremely detailed models of flowers made of painted glass. These were lampworked by Leopold Blaschka and his son Rudolph, who never revealed the method he used to make them. The Blaschka Glass Flowers are still an inspiration to glassblowers today. See the Harvard Museum of Natural History's page on the exhibit for further information.
Stained glass is an art form with a long history; many churches have beautiful stained-glass windows.
Glass in buildings
Glass has been used in buildings since the 11th century. Uses for glass in buildings include as a transparent material for windows, as internal glazed partitions and as architectural features.
Glass in buildings can be of a safety type, including wired, toughened and laminated glasses. Glass fibre insulation is common in roofs and walls. Foamed glass, made from waste glass, can be used as lightweight, closed-cell insulation.
Glass in vehicles
See also: sunroof, greenhouse, windshield.
Glass as a liquid
One arguably justifiable belief is that glass is a liquid of practically infinite viscosity at room temperature and as such flows, though very slowly, similar to pitch. Glass is generally treated as an amorphous solid rather than a liquid, though different views can be justified since characterizing glass as either 'solid' or 'liquid' is not an entirely straightforward matter . However, the notion that glass flows to an appreciable extent over extended periods of time is not supported by empirical evidence or theoretical analysis.
A myth does exist that glass rods and tubes can bend under their own weight over time. To check it, in the 1920s, Robert John Rayleigh, son of the Nobel Prize winner John William Rayleigh, conducted an experiment on a 1 metre (~39 in) long, 5 millimetre (~3/16 in) thick glass rod, which was supported horizontally on two pins with a 300 gram (~0.66 lb) weight in the middle. Apart from the initial bending of 28 millimetre (~1.1 in), the position of the weight did not change until the end of the experiment, which lasted for 7 years. At the same time, another man, a worker of General Electric named K. D. Spenser, conducted a similar experiment independently. Two months after Rayleigh, he published his own results which also disproved the myth. Spenser suggested that the myth was composed before the 1920s, when the tubes were made by hand, and naturally some of them were curved to begin with. Over time the straight tubes were taken away, and only the curved ones remained. Some people probably thought it was the glass flowing.
There is no clear answer to the question "Is glass solid or liquid?". In terms of molecular dynamics and thermodynamics it is possible to justify various different views that it is a highly viscous liquid, an amorphous solid, or simply that glass is another state of matter which is neither liquid nor solid.
Behaviour of antique glass
The observation that old windows are often thicker at the bottom than at the top is often offered as supporting evidence for the view that glass flows over a matter of centuries. It is then assumed that the glass was once uniform, but has flowed to its new shape.
The likely source of this belief is that when panes of glass were commonly made by glassblowers, the technique used was to spin molten glass so as to create a round, mostly flat and even plate (the Crown glass process, described above). This plate was then cut to fit a window. The pieces were not, however, absolutely flat; the edges of the disk would be thicker because of centrifugal forces. When actually installed in a window frame, the glass would be placed thicker side down for the sake of stability and visual sparkle. Occasionally such glass has been found thinner side down, as would be caused by carelessness at the time of installation.
Mass production of glass window panes in the early twentieth century caused a similar effect. In glass factories, molten glass was poured onto a large cooling table and allowed to spread. The resulting glass is thicker at the location of the pour, located at the centre of the large sheet. These sheets were cut into smaller window panes with nonuniform thickness. Modern glass intended for windows is produced as float glass and is very uniform in thickness.
Several other points indicate that the 'cathedral glass' theory is misconceived:
- Writing in the American Journal of Physics, physicist Edgar D. Zanotto states "...the predicted relaxation time for GeO2 at room temperature is 1032 years. Hence, the relaxation period (characteristic flow time) of cathedral glasses would be even longer" (Am. J. Phys, 66(5):392-5, May 1998). In layman's terms, he wrote that glass at room temperature is very strongly on the solid side of the spectrum from solids to liquids.
- If medieval glass has flowed perceptibly, then ancient Roman and Egyptian objects should have flowed proportionately more—but this is not observed.
- If glass flows at a rate that allows changes to be seen with the naked eye after centuries, then changes in optical telescope mirrors should be observable (by interferometry) in a matter of days—but this also is not observed. Similarly, it should not be possible to see Newton's rings between decade-old fragments of window glass—but this can in fact be quite easily done.
- Glass in refracting telescopes, with objective lenses of large diameter, are observed to sag under their own weight (causing a loss of focus), but this is due to elastic deformation and not because of the glass flowing over time; this (along with chromatic aberration and other effects) limits the size of refracting telescopes, with the largest refractor in the world being the Yerkes Observatory telescope with a diameter of 102 centimetres (40 in).
Comparison with pitch
Note that pitch, another seemingly-solid material, is in fact a highly viscous liquid, 100 billion times as viscous as water. This property can be seen in the University of Queensland's pitch drop experiment, where each drop has taken approximately 10 years to fall into the beaker.